IEC 81001-5-1: Cybersecurity during the life cycle of the Software of a Healthcare Product
The IEC 81001-5-1 regulation is a current regulation that is pending becoming harmonized standards and deals with the implementation of cybersecurity activities during the life cycle of software medical devices.
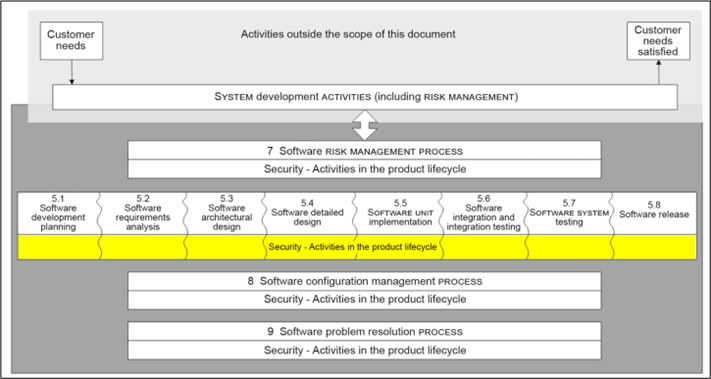
IEC 81001-5-1 defines the life cycle requirements for the development and maintenance of health software necessary to support compliance with IEC 62443-4-1, taking into account the specific needs of health software.
The set of processes, activities and tasks establishes a common framework for secure healthcare software lifecycle processes. The goal is to increase the cybersecurity of healthcare software by establishing certain activities and tasks in the healthcare software lifecycle processes and also by increasing the security of the software lifecycle processes themselves.
Contact an expert
If you want to know more about the topic or have any other type of question, do not hesitate, contact us.
Request more information
Follow us
Aviso Legal | Política de Cookies | Contacto
© 2025 Software Quality Systems S.A. | SQS is a member company of Innovalia





