EN 62366-1: Application of usability engineering to medical devices
This regulation is also one of those that was already among the harmonized standards in the old directives.
- Specifies a process for a manufacturer to analyze, specify, develop, and evaluate the usability of a medical device with respect to safety.
- This usability engineering process allows the manufacturer to evaluate and mitigate the risks associated with correct use and use errors, that is, normal use.
- It can be used to identify risks, but does not evaluate or mitigate the risks associated with abnormal use.
- This process is carried out in parallel to the risk management process.
A summary of the scope of this regulation is presented in the following image:
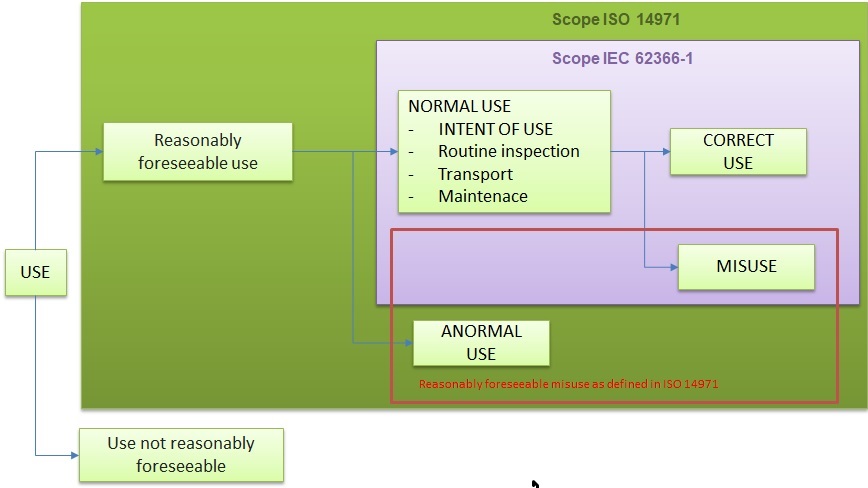
62366-1 usability
As can be seen, the scope of the usability regulation 62366-1 refers to the normal use of our medical device software.
The summary of activities to be carried out are the following:
- Usability engineering plan
- Usability input data
- Preparation specification of use
- Analysis
- Formative design and evaluation
- Summative evaluation
Contact an expert
If you want to know more about the topic or have any other type of question, do not hesitate, contact us.
Request more information
Follow us
Aviso Legal | Política de Cookies | Contacto
© 2025 Software Quality Systems S.A. | SQS is a member company of Innovalia





