EN 82304-1: Health software. Part 1: General requirements for product safety
The following standard, which we include in this article because it appears as a harmonized standard for obtaining the CE marking on medical devices, applies to the safety and security of health software products (the definition of health software product includes medical devices) designed to operate on general computing platforms and intended to be marketed without dedicated hardware. Its main focus is on the requirements for manufacturers. It covers the entire life cycle, including the design, development, validation, installation, maintenance, and disposal of health software products.
Examples of medical devices to which this regulation would apply:
- Mobile applications
- Standard PC applications
- Server applications
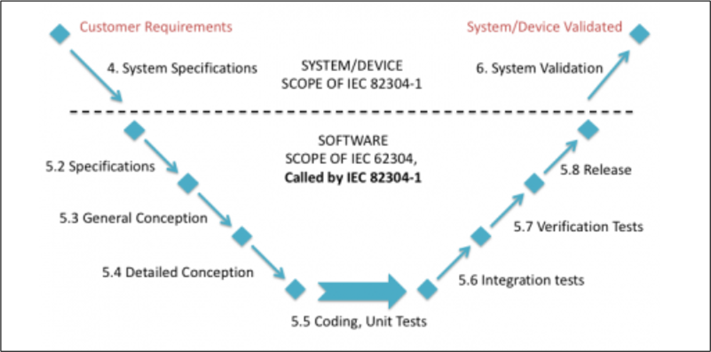
The following image shows the additional activities that are added to the IEC 62304 standard:
Contact an expert
If you want to know more about the topic or have any other type of question, do not hesitate, contact us.
Request more information
Follow us
Aviso Legal | Política de Cookies | Contacto
© 2025 Software Quality Systems S.A. | SQS is a member company of Innovalia





